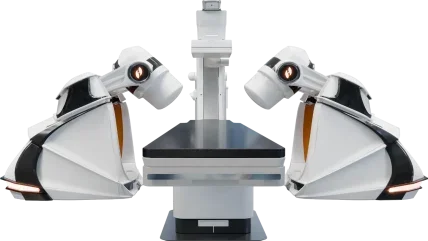
US-based surgical robotics manufacturer Stereotaxis has obtained the CE mark for its next generation robotic system, GenesisX, in Europe.
The company has also submitted a 510(k) application to the US Food and Drug Administration (FDA) for the same system.
Stereotaxis chairman and CEO David Fischel said: “Medical innovation only realises its full potential to advance and improve patient care if it is designed to be broadly accessible.
“The clinical value of Stereotaxis’ robotic technology has been demonstrated yet is difficult to access for many physicians and hospitals. GenesisX supports broader adoption of robotics in electrophysiology and endovascular interventions.”
According to Stereotaxis, GenesisX builds on the established benefits of robotic magnetic navigation (RMN) systems while aiming to reduce the complexities of hospital adoption.
Stereotaxis noted that preparing an operating room for an RMN system traditionally requires significant structural modifications, such as installing magnetic shielding, reinforcing the floor, and running extensive cabling. This involves considerable planning and coordination among site planners, architects, and contractors.
In contrast, the GenesisX system simplifies this by using smaller magnets and incorporating magnetic shielding into the system itself, thus eliminating the need for additional shielding in the operating room walls. It also avoids structural modifications by not requiring floor anchoring and operates on standard 120/230V power outlets.
A single fibre cable connects each robot to a system cabinet, which is 80% smaller than the previous Genesis model’s cabinet and can be placed under a table in the operating room.
Despite being smaller and lighter than previous models, GenesisX is said to maintain the speed, responsiveness, and workflow of its predecessor and is intended to serve as a platform for future innovations.
Stereotaxis plans to focus on obtaining regulatory approval for compatible catheters. The company will also demonstrate the system’s real-world use and work on enhancing compatibility with various x-ray systems.
Additionally, Stereotaxis will prepare its supply chain, manufacturing, installation, and commercial processes for the planned launch and adoption of GenesisX in 2025.
The New York Stock Exchange (NYSE) listed company also announced its financial results for Q2 2024 ended 30 June 2024. Revenue for the quarter totalled $4.5m, down from $7.9m in the same period last year.
System revenue, which includes income from the sale and installation of new robotic navigation systems, was $0.2m, compared to $3.3m in the prior year’s second quarter.
Recurring revenue, from ongoing service contracts and maintenance, was $4.3m, compared to $4.6m in the same period last year.
Stereotaxis said that the decline in system revenue was due to delays in hospital construction schedules and does not reflect the company’s typical expectations.






