Target Market

We continue to invest heavily in the ABC audited circulation of Medical Device Developments and have created the most exciting database available. Copies are sent by name to all key decision-makers in the medical device industry, which will generate a projected readership of approximately 41,000 (publisher's survey), accounting for the majority of all purchasing activity by the medical device OEM community.
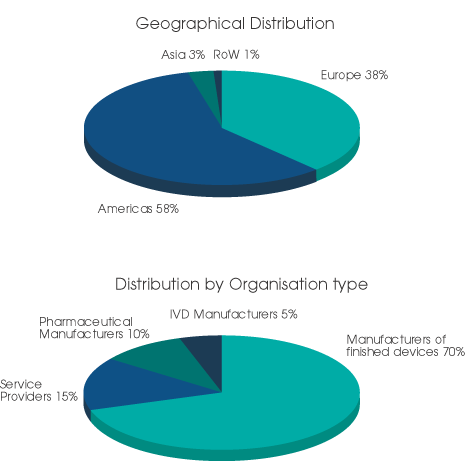
Readers of Medical Device Developments include individuals
responsible for the following activitiesTop
| R&D |
| Product design / engineering |
| Production / manufacturing |
| Quality control / assurance |
| Regulatory affairs |
| Corporate / general management |
| Packaging |
| Purchasing |
| Project / process engineering |
Within organisations such asTop
| Johnson & Johnson |
| B Braun Melsungen AG |
| Cordis Corporation |
| Tyco Healthcare Group |
| Stryker Corporation |
| Becton, Dickinson & Company |
| GE Healthcare |
| Abbott Laboratories |
| GlaxoSmithKline PLC |
| Siemens Medical Systems |
| Baxter International Inc |
| Bayer Healthcare AG |
| Agfa-Gevaert N.V. |
| Smith & Nephew PLC |
| Drager Medical AG & Co KGaA |
Representative job titles include Top
| Head of Supply Chain |
| Head of Logistics |
| Head of Operations |
| Head of Product Design |
| Head of Research & Development |
| Senior Process Engineer |
| CEO |
| Managing Director |
| President |
| Senior Packaging Engineer |
| Purchasing Director |
| CIO |
| Quality Control Manager |
| Technical Director |
| Plant Managers |
| IT Director |
| Heads of Quality Control |
| Procurement Manager |

